Phosphate is indeed a polyatomic ion, consisting of the phosphorus atom bonded to four oxygen atoms. Let’s take a closer look at what exactly phosphate is and why it is classified as a polyatomic ion.
What is a Polyatomic Ion?
Before discussing phosphate specifically, it’s helpful to understand what a polyatomic ion is.
A polyatomic ion is a grouping of multiple atoms that carries an overall electric charge. The key criteria are:
- It consists of two or more atoms
- The atoms are chemically bonded together
- The group carries a net positive or negative charge
Some common examples of polyatomic ions include:
- Nitrate NO3- – containing nitrogen and oxygen, with a charge of -1
- Ammonium NH4+ – containing nitrogen and hydrogen, with a charge of +1
- Carbonate CO3- – containing carbon and oxygen, with a charge of -2
Polyatomic ions act as a single unit, almost like an element. But unlike a monatomic ion, which contains only one atom, a polyatomic ion has that distinctive multiple atom structure.
The charges on polyatomic ions can vary, depending on the number of extra electrons or electron deficiencies. But whatever the charge, the entire polyatomic ion takes on that charge.
Now that we have defined what a polyatomic ion is, we can analyze phosphate to see if it fits the criteria.
The Phosphate Ion Composition
Phosphate consists of one phosphorus atom bonded to four oxygen atoms and carrying a negative three charge, written as PO43-.
Breaking this formula down tells us:
- It contains multiple atoms – one P and four O’s
- These atoms are chemically bonded together through covalent bonds
- The entire ion carries a charge of -3
Therefore, phosphate satisfies all the requirements to be classified as a polyatomic ion. The phosphorus atom forms strong covalent bonds with the four oxygen atoms, which stick together as a single functioning unit.
Let’s look a little closer at the structure and characteristics of the phosphate ion.
Phosphate Structure
The phosphate ion has a tetrahedral structure, with the phosphorus atom situated in the middle surrounded by four oxygen atoms at the corners.
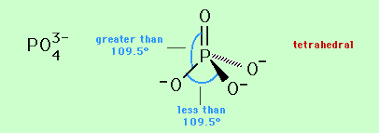
The tetrahedral structure of phosphate.
This tetrahedral arrangement minimizes repulsion between the negatively charged oxygen atoms, providing stability. The phosphorus and oxygen have a covalent bond order of 1, with 3 unshared electron pairs on the phosphorus.
Oxidation States
Phosphorus is capable of forming several different oxidation states, including phosphates with different numbers of oxygen atoms. Some examples include:
- Orthophosphate PO43-
- Pyrophosphate P2O74-
- Triphosphate P3O105-
But the most common and prevalent form is orthophosphate, with phosphorus in a +5 oxidation state. This PO43- form dominates biological systems and is the most stable.
Presence and Importance
Phosphate plays crucial roles throughout nature, including:
- Forming the backbone of DNA and RNA molecules
- Providing structure to cell membranes via phospholipids
- Playing essential roles in energy storage and metabolism as ATP
- Making up about 1% of the human body weight, mostly in bones and teeth
Phosphorus is actually the 11th most abundant element in the human body. Phosphate esters also show up frequently in important biological molecules.
Clearly phosphate has widespread significance across many domains of chemistry and biology. And central to all of these critical functions is its structure as a polyatomic ion.
How Does Phosphate Achieve a -3 Charge?
As we’ve seen, phosphate has an overall charge of -3. But where does this negative charge come from?
It stems from the difference in electronegativity between the phosphorus and oxygen atoms. Oxygen is substantially more electronegative than phosphorus, meaning it has a stronger pull for shared electrons in the covalent bonds between them.
This electronegativity difference pulls the shared electrons closer to the oxygen atoms, giving them a partial negative charge.
With four oxygen atoms each taking on a partial negative charge, these add up to an overall charge of -3 for the complete phosphate ion.
The phosphorus atom takes on a corresponding +3 partial positive charge, though this is offset by the negative charges on the oxygens.
- Why Are Javan Rhinos Going Extinct?
- Is Bonaire Sint Eustatius and Saba a Country?
- Why Is Believing In Yourself So Important?
How Phosphate Differs from Phosphoric Acid
Phosphate and phosphoric acid have similar chemical compositions but differ in their structures and properties. It’s important to distinguish between them.
Phosphoric acid has the chemical formula H3PO4. It consists of one phosphorus atom, four oxygen atoms, and three hydrogen atoms.
Unlike phosphate, phosphoric acid…
- Does not carry an overall electric charge
- Has hydrogen atoms bonded to oxygen atoms
- Can dissociate to release H+ protons
Phosphoric acid acts as an acid – it is capable of releasing H+ ions into solution and donating protons. This gives it an acidic pH when dissolved in water.
Phosphate, on the other hand:
- Carries a negative 3 charge
- Does not contain any hydrogen atoms
- Cannot donate protons or act as an acid
So while their compositions are similar, phosphoric acid and phosphate ions have very different properties and reactivities. Phosphate does not display acidic behavior.
How Phosphate Relates to Phosphorus Pentoxide
Phosphorus pentoxide, also called phosphoric anhydride, has the chemical formula P4O10. It can react with water to produce phosphate ions.
Phosphorus pentoxide consists of 4 phosphorus atoms bonded together in a lattice structure, with oxygen atoms bridging between the phosphorus. Each phosphorus atom is bonded to 5 oxygen atoms.
When phosphorus pentoxide dissolves in water, its phosphorus-oxygen bonds break. Each phosphorus ends up surrounded by 4 oxygen atoms, forming phosphate ions:
P4O10 + 6H2O → 4PO43- + 10H+
So even though their compositions differ, phosphorus pentoxide can readily produce phosphate ions upon dissolution in water. This reaction with water to generate phosphate is useful in producing fertilizers.
- How Much Is Lvn Program at Concorde?
- Why Do Cowboys Have So Much Trouble with Math?
- Who Invented the Airfoil? A Historical Summary of Key Contributions
Common Phosphate Compounds
Phosphate polyatomic ions show up in many different compounds, both inorganic and organic. Some examples include:
- Calcium phosphate – The main mineral component of bones and teeth
- Sodium phosphate – An ingredient in detergents and food additives
- Guanosine monophosphate (GMP) – A nucleotide found in RNA
- Adenosine triphosphate (ATP) – The main energy molecule in cells
In these compounds, phosphate acts as a distinct structural unit that retains its polyatomic identity. This reiterates how phosphate behaves as a single ionic group even when part of larger molecules.
Phosphate: A Vital Polyatomic Ion
In summary, phosphate (PO43-) matches the definition of a polyatomic ion in every way:
- It is comprised of multiple atoms – 1 phosphorus and 4 oxygens
- These atoms are covalently bonded together
- The ion as a whole carries a negative charge of 3-
Phosphate’s abundant presence throughout biology and biochemistry relies heavily on its tetrahedral structure and -3 charge. Without its polyatomic properties, phosphate could not fulfill so many crucial roles across diverse organic and inorganic compounds.
So phosphate stands as an exemplary model of a polyatomic ion – a multi-atom grouping with a net charge that behaves as a single unit. This combination of attributes makes phosphate essential to life processes and demonstrates why polyatomic ions like phosphate are so important.
- Is Balloon a Gas?
- Why Is Believing In Yourself So Important?
- Does Undigested Food Cause Constipation?
- What Does “Slow Time” Mean?
- How to Tow a Ford F150 4×4?
- How Many Jobs Are Available in Edp Services?
- Are Dementia Patients Violent?
- What Does the Wrench Light Mean on a Ford F150?
- Was Brandon from Glow Up on Botched?
- Why Does DoorDash Keep Pausing?
- Can You Lactate Without Being Pregnant?
- How to Remove a Tick from a Dog? [Beginners Guide]
- Does It Snow in Halifax?
- Where Is Miyagi Dojo?
- Ingredients in Kingsford Charcoal?