Key Takeaways:
- Globular proteins are spherical or globe-like proteins that play various roles in organisms.
- They are water-soluble and have complex 3D structures with alpha-helices and beta-sheets.
- The compact folding of the amino acids gives globular proteins their rounded shape.
- Examples include hemoglobin, albumin, myoglobin, and antibodies.
- Misfolding of globular proteins is linked to diseases like Alzheimer's and Parkinson's.
- Is Phosphate a Polyatomic Ion?
- What is Prograde and Retrograde Rotation?
- Why Are Javan Rhinos Going Extinct?
Introduction
Proteins are essential biomolecules that perform a wide array of functions within living organisms. They are comprised of chains of amino acids that fold into specific three-dimensional structures. These structures enable proteins to interact with other molecules in the body and carry out specific roles. One of the most common classes of proteins is globular proteins. But what exactly are globular proteins?
This comprehensive article will analyze globular proteins in detail – their structure, function, location, disease relevance, and more. The breadth of information provided will help readers gain a deeper understanding of the significance of these spherical proteins in biology. Discover what makes globular proteins unique, examples of important globular proteins, and how their misfolding can lead to serious health conditions. This guide aims to answer common questions and provide a thorough overview of globular proteins so readers can grasp their crucial biological purposes.
The intricacies of protein folding and how the spherical shape of globular proteins is achieved will be explored. Additionally, the article highlights relevant scientific studies and statistics to underscore the impact of continued research into these proteins. Readers will leave with an enriched knowledge of what globular proteins are composed of, how they are formed, where they are found, their health implications, and more. Let's begin unraveling the world of globular proteins!
What Is the Structure and Composition of Globular Proteins?
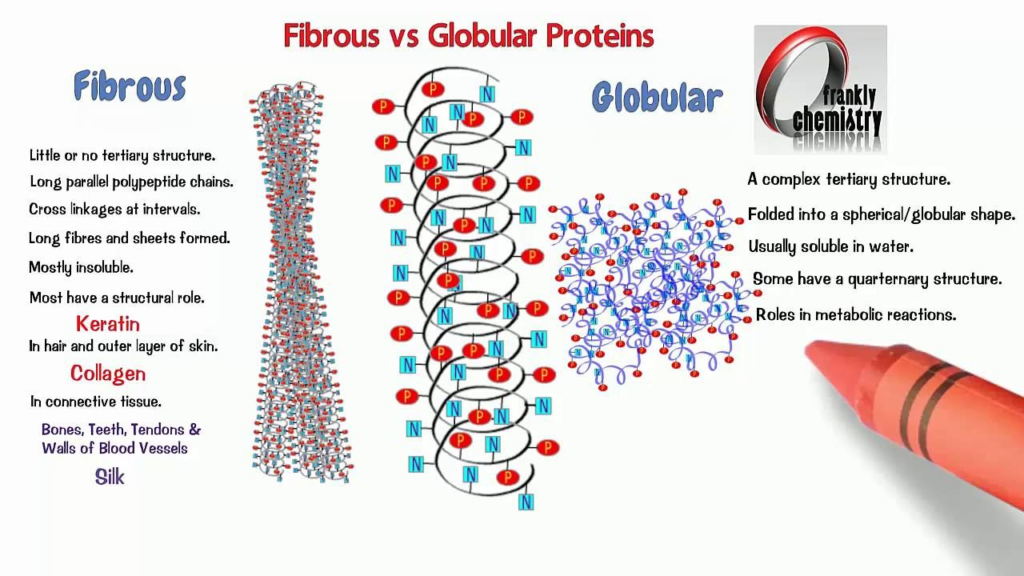
Globular proteins get their distinct spherical shape from the unique way their long, looping polypeptide chains fold and compress together. But what causes these proteins to fold into a compact ball?
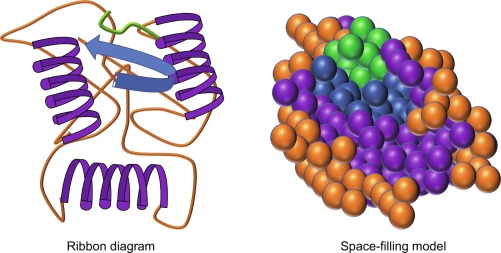
How Do Amino Acids Fold Into a Globular Shape?
The sequencing and bonding of the amino acids in the polypeptide chains allows the long protein molecule to fold in on itself in certain areas. This creates the rounded, globular shape.
Specifically, globular proteins contain large amounts of alpha-helix and beta-pleated sheet secondary structures within their amino acid sequences. The alpha helix segments coil while the beta sheets align in a pleated, rippled pattern.
This allows the long protein strand to twist and fold in a very compact way, resulting in the spherical globular shape rather than an elongated fibrous structure. The globular structure allows these proteins to be soluble.
What Bonds Maintain the Globular Shape?
Several types of chemical bonds help maintain the folded globular conformation:
- Hydrogen bonds – Form between the partially positive hydrogen atom and partially negative oxygen/nitrogen atoms on the amino acid backbone. This provides stability to the secondary structures.
- Ionic bonds – Form between oppositely charged amino acid side chains (R groups). Help hold tertiary structure in place.
- Disulfide bridges – Covalent bonds between sulfur atoms on cysteine residues. Like glue that holds the final shape together.
- Hydrophobic interactions – Clustering of nonpolar R groups in the interior away from water. Further compacts the protein.
These various chemical interactions all work together to keep the long amino acid chain folded up into the spherical globular shape.
- Are Biologics Made from Stem Cells?
- What Is a Sentence for Suboceanic?
- Did Henry Ford Start Kingsford Charcoal?
Where Are Globular Proteins Found and What Roles Do They Play?
Now that the composition and folding of globular proteins is understood, where are these spherical molecules found and why are they important?
In Which Parts of the Cell/Body Are Globular Proteins Located?
Globular proteins are found throughout the body:
- Cytoplasm – As free-floating proteins performing various metabolic functions and enzymatic reactions.
- Blood plasma – Examples are serum albumin and antibodies. Help regulate osmotic pressure and transport substances.
- Cell membrane – Assist in cell signaling, cell adhesion, ion transport, etc.
- Extracellular fluid – Act as messengers, antibodies, carriers of lipids/minerals.
- Storage – In egg whites, milk, and intracellular sites. Provide nutrient reserves.
So globular proteins are located both inside cells and extracellularly to conduct critical biological processes.
What Key Functions Do Globular Proteins Perform?
Globular proteins are involved in nearly every physiological process due to their versatility. Some vital roles include:
- Enzymes – Catalyze metabolic reactions as globular enzyme proteins. Example: pepsin.
- Messengers – Transmit signals to coordinate biological processes. Example: insulin.
- Movement – Muscle contraction occurs through myosin/actin protein interactions.
- Immune function – Antibodies that target antigens are globular immunoglobulins.
- Transport – Hemoglobin transports oxygen in blood. Lipoproteins carry lipids.
- Structural support – Tubulin forms microtubules that support cell shape.
- Regulation – Albumin regulates blood osmotic pressure.
The variety of critical tasks performed by globular proteins highlights their importance in sustaining life. Their soluble nature and ability to fold into specialized shapes allows them to conduct such a multitude of roles.
Examples of Important Globular Proteins and Their Biological Functions
To understand their significance, let's explore some well-known examples of globular proteins and the vital jobs they carry out:
Hemoglobin – Oxygen Transport
Hemoglobin is a globular protein found within red blood cells. Each hemoglobin molecule contains four myoglobin protein subunits, each with a ferrous heme group that can bind oxygen. This gives hemoglobin the ability to carry oxygen from the lungs to body tissues. The unique globular structure changes shape to bind/release oxygen as needed.
Myoglobin – Oxygen Storage
Myoglobin is composed of a single polypeptide chain that folds into a compact globular shape. The heme group, similar to hemoglobin, allows myoglobin to bind oxygen. It is found in muscle cells where it stores and supplies oxygen to enable cell respiration during muscle contraction. The globular structure allows soluble myoglobin to diffuse through the sarcoplasm.
Enzymes – Metabolic Reactions
Enzymes like pepsin, amylase, and lipase adopt complex globular conformations that give them specific active sites and functions. Their globular shapes allow substrate molecules to bind to the active sites and undergo catalysis. This accelerates essential metabolic reactions.
Immunoglobulins – Immune Response
Antibodies like IgG and IgE have globular structures formed from multiple peptide chains. This shapes the paratope region that binds to specific pathogen antigens. This stimulates an immune response against foreign invaders. The globular shape allows flexibility in binding diverse antigens.
Insulin – Blood Glucose Regulation
Insulin is a globular protein hormone secreted by pancreatic beta cells. It consists of two peptide chains linked by disulfide bonds in a compact rounded shape. Insulin binds to cell receptors to stimulate glucose uptake and regulate blood sugar levels. The globular structure allows insulin to move freely through the bloodstream.
So we see diverse examples of critical globular proteins – from oxygen handling, to metabolism, to immune function, and hormone regulation. Their special globular conformations enable such life-sustaining biological roles.
- Will the UK Build a Third Aircraft Carrier?
- Are the Sides of a Right Angled Triangle Equal?
- What Does Suprachoroidal Mean?
How Does Protein Folding Relate to Diseases Like Alzheimer's?
While proper folding is crucial to globular protein function, misfolding can occur and is related to several diseases:
What Causes Protein Misfolding?
Mutations, cell stress, pH changes, molecular crowding, etc. can alter amino acid interactions, disrupting folding. Non-native structures result.
Misfolded proteins are unable to function properly and often clump together. These aggregates lead to cellular toxicity and pathology.
What Diseases Are Linked to Globular Protein Misfolding?
- Alzheimer's – Amyloid plaques formed by misfolded beta amyloid proteins. Leads to dementia.
- Parkinson's – Alpha-synuclein protein misfolding causes Lewy body aggregates damaging neurons.
- Cystic fibrosis – Delta F508 mutation in CFTR protein causes misfolding and lost chloride ion transport.
- Mad Cow disease – Misfolded prion proteins lead to neurodegeneration.
- Huntington's disease – Mutant huntingtin protein misfolds and accumulates in the brain.
- Amyloidoses – Deposits of misfolded proteins disrupt organ functions.
Understanding how alterations in globular protein folding equates to toxicity and dysfunction provides insight into developing targeted treatments. Correcting misfolding could prove therapeutic for many diseases.
Researchers at the University of Cambridge have been able to design anti-cancer ligands that can bind folded but not misfolded versions of globular proteins. This selectivity for properly folded conformations has potential for treating diseases like Alzheimer's and Parkinson's by targeting misfolded proteins (Pandya, 2021). Further innovations in exploiting differences in protein folding may yield clinical breakthroughs.
Key Questions About Globular Proteins
What Causes the Round Shape of Globular Proteins?
The round, spherical shape of globular proteins is caused by the folding and coiling of their polypeptide chains into alpha-helices and beta-pleated sheets. This allows the long protein molecule to twist into a compact, globular conformation stabilized by hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions.
Where Are Globular Proteins Located?
Globular proteins are located throughout the body – in the cytoplasm, blood plasma, cell membranes, extracellular fluid, and in storage. Their soluble nature allows them to take on numerous structural and functional roles.
Why Are Enzymes Globular?
Enzymes need to be globular to function. Their specific 3D shapes give enzymes their active sites that substrate molecules can bind to. The globular structures also allow flexibility and mobility to interact with substrates. A compact, soluble globular shape is essential for enzymes to catalyze biochemical reactions efficiently.
How Does Hemoglobin Transport Oxygen?
Hemoglobin contains iron ions that enable each subunit to bind oxygen molecules. The globular shape allows hemoglobin to capture oxygen in the lungs and transport it safely through the bloodstream to release at body tissues. Conformational changes in the globular structure assist in oxygen loading and unloading.
Why Must Antibodies Have a Globular Structure?
Antibodies like IgG and IgE require a globular structure to be able to recognize and bind to diverse antigens. The Y-shaped globular form provides two antigen-binding sites on the antibody. The rounded compact structure also gives antibodies stability and solubility to circulate in the blood and penetrate tissues to find foreign invaders.
- What Does Cordate Leaf Mean?
- What Is a Third Class Degree?
- Where Did Utnapishtim Live? An In-Depth Examination of the Mysterious Location
Conclusion
In summary, globular proteins are a ubiquitous and highly diverse class of proteins that adopt a spherical, compact shape due to their structural folding and bonding patterns. They are located throughout the body and carry out a vast array of essential biological functions including enzyme activity, immune defense, oxygen transport, cell signaling, and structural support.
Their soluble globular conformations allow these proteins to traverse cellular and extracellular environments and interact with various molecules to conduct their physiological roles. However, mutations and other factors can disrupt proper folding, leading to protein aggregation and diseases like Alzheimer's.
This article provided an extensive overview of globular protein composition, structure, function, examples, disease relevance, and other key properties that distinguish these spherical biomolecules. The abundance of critical globular proteins highlights their importance in sustaining life. With further research, these remarkable structures may hold keys to treating currently incurable protein misfolding diseases.
- How to Weatherize a TV for Outdoor Use?
- How to Disengage 4 Wheel Drive on Ford F150?
- Does Two Faced Lip Injection Hurt?
- Can I Wear Invisalign in the Shower?
- Can l5-s1 Cause Constipation?
- Where Did 0 Degrees Fahrenheit Come From?
- does costco sell pickleball paddles?
- Which Are the Illuminating Parts of the Microscope?
- How to Remove Ford F150 Bed Cover? A Step-by-Step Guide
- How Much Is a Sleep Number 360 Smart Bed Cost?
- How to Watch Impractical Jokers on Hulu?
- What Is Sake Lees? An In-Depth Look at This Unique Byproduct of Sake Making
- How to Download OnlyFans Videos on Android?
- Where Is Parenthood Streaming? An In-Depth Look at the Best Platforms to Stream This Beloved Family Drama