The lysogenic phage is a fascinating biological entity that plays an important role in shaping microbial ecosystems. This comprehensive guide will explore all aspects of the lysogenic phage, from its unique infection process to its effects on bacterial hosts. Read on to discover the answers to common questions about this temperate virus.
Key Takeaways:
- The lysogenic phage can integrate its DNA into the host cell's genome and replicate along with it.
- Instead of immediate destruction, the lysogenic cycle allows dormancy inside the host cell.
- Environmental stresses like nutrient depletion can trigger the lysogenic phage to enter the destructive lytic cycle.
- Lysogenic phages provide advantages to host cells by conferring viral genes with helpful functions.
- Shifting between lytic and lysogenic cycles allows phages to persist and affect bacterial populations.
- How to Become a Company Man in the Oilfield?
- Who Gave All Might His Power?
- Did the Pilgrim Fathers Sail from Boston Lincolnshire?
What Exactly Is a Lysogenic Phage?
A lysogenic phage, also known as a temperate phage, is a type of virus that infects bacteria. Upon infection, the lysogenic phage integrates its own genetic material into the bacterial cell's chromosome and replicates along with the host DNA. This allows the virus to remain dormant inside the cell in a lysogenic cycle without destroying it right away. The phage nucleic acid may later be induced to enter the lytic cycle, where it replicates rapidly and lyses the host cell.
Lysogenic phages are distinct from strictly lytic phages that immediately enter the lytic cycle upon infection and cause the bacterial cell to burst. The ability to shift between replication modes provides unique advantages to lysogenic phages and their hosts. These fascinating viruses blur the line between symbiosis and parasitism in their complex relationships with bacteria.
What Is the Lysogenic Cycle of Phage Infection?
The lysogenic cycle is characterized by the following key steps:
1. Injection
The phage attaches to receptors on the bacterial cell surface and injects its nucleic acid (DNA or RNA) into the host through its protein shell.
2. Integration
Once inside the cell, the phage nucleic acid integrates into the bacterial chromosome with the help of enzymes called integrases that are encoded by the phage. This forms a prophage that can now replicate passively along with the host DNA.
3. Replication
During subsequent cell divisions, the incorporated prophage gets copied and passed on to new generations of cells. The phage DNA essentially becomes part of the bacterium's genome.
4. Dormancy
Instead of taking over the cell's mechanisms like a lytic phage, the prophage remains dormant and does not immediately cause any harm to the host. This allows both organisms to subsist.
5. Induction
Under certain environmental stresses like depletion of nutrients, damage to DNA, or introduction of mutagens, the lysogenic phage can be induced to exit dormancy. Enzymes are activated that excise the viral genome from the host DNA.
The prophage then enters the lytic cycle, where it rapidly replicates, produces new phage particles, and lyses the host cell to release mature virions. This ends the lysogenic state.
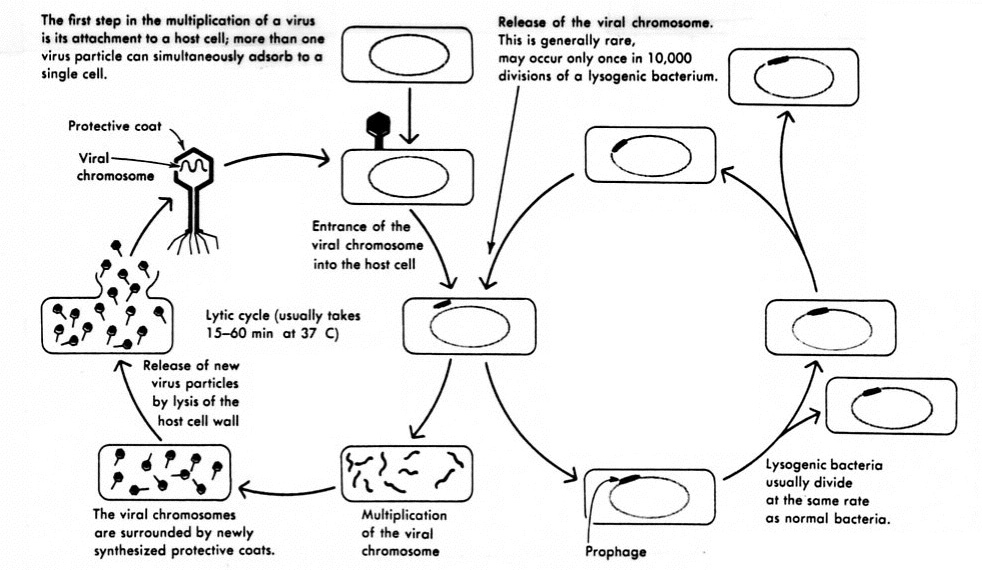
What Are the Benefits of Lysogeny for Phages?
The lysogenic lifecycle confers several key benefits that enhance the fitness and survival chances of the infecting phage:
- Refuge from harsh conditions: Inside the host cell, the phage is protected from environmental factors like radiation, antibiotics, or disinfectants that could inactivate its particles outside.
- Vertical transmission: Integration allows the phage genome to be passed on to daughter cells along with the host DNA during binary fission. This vertical transmission to new host cells occurs with zero energy expenditure.
- Persistence in host: Avoiding immediate lysis of the host enables the phage to remain associated for multiple generations. This increases its chances of induction and spread under favorable conditions.
- Genetic variation: Occasional mutations in the incorporated prophage can produce new phenotypic traits and lead to phage evolution.
- Gene transfer: Prophage genes can sometimes provide fitness benefits to bacterial hosts, allowing mutualistic relationships to develop.
By alternating between lysogenic and lytic modes, phages can persist in bacterial communities and withstand adverse circumstances. The prophage state serves as a reservoir for phage survival.
How Might Lysogeny Benefit the Infected Bacteria?
The lysogenic state is not always parasitic to the host cell. Some key potential benefits for bacteria include:
- Immunity: A prophage can confer immunity against superinfection by the same or closely related phages, protecting the cell.
- Beneficial genes: Lysogenic phages occasionally provide useful viral genes that can enhance host fitness. These may code for toxins, enzymes, or metabolites that help the bacterium adapt and compete.
- Survival: Avoiding immediate lysis allows the host cell to remain viable and propagate normally for several generations.
- Metabolic genes: Certain prophages contain genes that benefit host cell metabolism by allowing utilization of new substrates.
- Stress tolerance: Some evidence indicates prophages help bacteria withstand stressors like starvation, antibiotics, and immune responses.
- Virulence factors: Prophage encoding of toxins or surface proteins can increase bacterial virulence and pathogenicity in diseases.
Lysogeny does not benefit all hosts, but temperate phages and bacteria likely evolve symbiotically at times, leading to positive or benign effects of phage integration. Both organisms can enhance their survivability through their intricate relationship.
What Triggers Induction of Lysogenic Phages?
Lysogenic phages typically remain dormant within the host cell for multiple generations. However, certain conditions can trigger their excision from the bacterial chromosome and entry into the lytic cycle. Known inducing agents include:
- DNA damage: Exposure to ultraviolet radiation or mutagenic chemicals can instigate the SOS response and prophage induction.
- Nutrient depletion: Starvation signals like absence of critical nutrients (such as carbon, nitrogen, or phosphorus) may switch phages into the lytic mode.
- Temperature: Increased temperature can directly denature phage repressor proteins and de-repress the phage transcriptional switch.
- Oxidative stress: Reactive oxygen compounds that disrupt thiol groups essential for repressor functioning may induce excision.
- Antibiotics: Antibiotics like quinolones and beta-lactams can activate lytic growth by generating cellular stress signals.
- Spontaneous induction: Random stochastic events and mutations in phage repressors provide a low baseline rate of spontaneous induction.
Environmental perturbations that destabilize host cells commonly act as triggers. By monitoring host physiology, lysogenic phages time their excision and lytic activities for optimal reproductive success.
How Does the Lytic Cycle of Phages Differ?
In contrast to the lysogenic pattern, the lytic cycle of bacteriophages is characterized by rapid self-replication and cell lysis:
- Upon infection, the phage commandeers the host machinery and diverts it towards production of new phage components.
- It degrades the bacterial genome while synthesizing numerous copies of its own DNA/RNA along with structural proteins.
- These components self-assemble into fresh phage progeny within minutes to hours, often numbering in the hundreds.
- Holin and lysozyme enzymes expressed late in infection break open the cell wall, releasing the mature virions to infect new hosts.
- The burst size per cell ranges from tens to hundreds for different phages.
- The entire process quickly kills the host, which is unable to sustain this parasitic assault.
Lytic phages thus amplify themselves exponentially at the cost of bacterial viability. Lysogeny allows a more balanced co-existence.
How Do Lysogenic Phages Switch Between Replication Modes?
The decision between entering lysogeny or the lytic pathway depends on multiple factors:
- Phage genes: Key regulator and recombinase genes influence the establishment and maintenance of lysogeny after injection.
- Host physiology: The physiological state of the bacterium affects whether phages opt for dormancy or immediate lysogenic reproduction.
- Multiplicity of infection: Higher viral loads favor lytic growth, while lower rates increase lysogenic responses.
- Environmental stimuli: Stressors like starvation, antibiotics, UV damage, and temperature shifts can modulate lytic induction.
During stable conditions, lysogeny prevails through phage repressors that silence lytic genes. Stressors often de-repress lysogenic phages as resources decline and lysis offers the best chance for transmission. Complex phage-host interactions underlie shifts between the cycles.
What Effects Can Lysogeny Have on Microbial Communities?
Lysogenic phages are influential drivers of bacterial evolution and community dynamics:
- Phage-mediated horizontal gene transfer between different bacterial strains and species via transduction can spread beneficial traits like antibiotic resistance.
- Immunity in lysogenic cells limits spread of superinfecting phages, generating specific resistance. This selects for phage mutants capable of circumventing immunity.
- Virulence enhancement by prophage encoding of toxins and enzymes like Shiga toxin or diphtheria toxin can increase bacterial pathogenicity.
- Biofilm disruption through prophage induction can cause lysis of matrix-associated cells and dispersal of progeny to new sites.
- Microdiversity arises within lysogens as prophages impart variable fitness effects. This fuels competition between phage-infected lineages.
- Stress tolerance gained through some prophages may provide hosts a selective advantage during adverse conditions.
Lysogenic conversion and shifts to lysis generate substantial phage-mediated diversity that fuels bacterial adaptation and ecosystem-level changes.
Can Lysogenic Phages Be Harnessed for Useful Applications?
The unique properties of temperate phages offer intriguing possibilities for bioengineering and therapy:
- Phage typing: Prophage-mediated resistance patterns help fingerprint bacterial strains for epidemiological monitoring.
- Recombinant protein production: Harmless prophages are engineered to carry genes expressing desired proteins like insulin.
- Vaccines: Inactivated prophages containing toxin genes provide protection by eliciting neutralizing antibodies.
- Gene delivery: Phage vectors transport desirable genes like CRISPR-Cas into challenging bacterial recipients.
- Antibacterial agents: Lysogenic induction or engineered lytic repression failure provide ways to selectively eliminate pathogens.
- Microbiome manipulation: designed prophages may allow targeted reshaping of complex native communities.
When mastered, lysogenic phages could enable a medicine chest of tailored applications that control bacteria for our benefit.
Conclusion
Lysogenic bacteriophages have intricate lifecycles that involve integration into host chromosomes and shifts between latent and lytic modes. As both temporary refuges and opportunistic parasites, they walk a fine line between symbiosis and predation with their bacterial hosts. Lysogeny's effects cascade through microbial communities, driving evolution and ecological dynamics. Further research into the molecular triggers underlying lysogenic induction will uncover new ways to harness temperate phages for therapeutic and biotechnological innovations that can solve problems and improve lives.
- Can You Use Apple Pay on DoorDash?
- Are Jesus and Yahweh the Same?
- Why Is Every 4th Year a Leap Year?
- How Much Does an Abortion Cost in Missouri?
- How To Replace Ford F250 Windshield Wiper Motor?
- Why Did Monster Woo Go to Jail?
- Why Is My Hamster Walking Slowly?
- Are We in a Recession? Detailed Guide
- Will My Cat Get Along with a Kitten?
- How to Change Front Wheel Bearing on Ford F350 4×4?
- Why Didn't the Plant Work on Ray in Bloom?
- Why Are You Doing This Duke Manga?
- What Is a Counter Statement?
- How to Beat a Controlled Buy?
- Can you take a pickleball paddle on a plane?